news, webinars, whitepapers, & more
Resources

A Closer Look at the New Annex 1: What’s included and What It Means for the Fill-Finish Industry
With the revised Annex 1 coming into effect last year, the pharmaceutical industry continues to strategize the best way to attain and maintain compliance with the intensive regulation on sterile medicinal products. Aseptic fill-finish manufacturing, for its part, is established on precise science and the highest standards of safety. While much of the science, technology,…
AST Revolutionizes Fill-Finish: American Pharmaceutical Review’s interview with Josh Russell
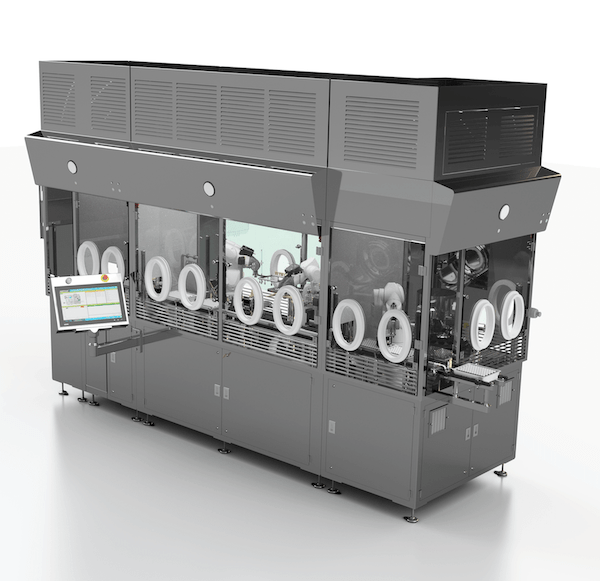
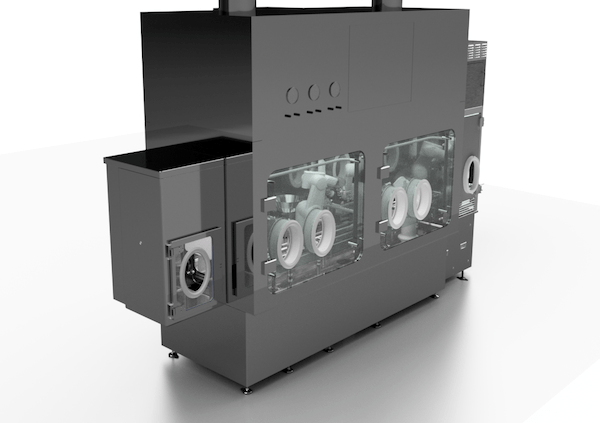
Josh Russell, Vice President of Sales and Marketing, recently spoke to American Pharmaceutical Review on AST’s latest aseptic processing solutions. American Pharmaceutical Review, a leading journal for business and technology, connected with Josh at Interphex 2024, where AST exhibited our latest generation GENiSYS® C housed inside AST’s new isolator solution. The exhibit showcased the latest…
Virtual Pharma Expo 2024: Fill-Finish Configurations for Cell & Gene Therapies
Josh Russell, AST’s Vice President of Sales and Marketing, recently took part in the Virtual Pharma Expo: Aseptic Fill/Finish, Manufacturing and Packaging with a walkthrough of some of the latest AST technology, demonstrated on our GENiSYS® R robotic line.
Highlighting Innovation: A Video Breakdown of AST’s New Fill-Finish Isolator
AST’s Director of Engineering Spencer Bolte walks through some of the key design considerations on AST’s new isolator including operator-centered solutions and a groundbreaking new decontamination technology for complete 60-minute cycle times, including aeration. https://www.youtube.com/watch?v=0xlKE43pl2U The newly unveiled AST isolator and the latest generation GENiSYS C are two of AST’s intuitive, compact solutions for high-value…
Steven Ng Discusses AST’s New Isolator Solution with Cleanroom Technology
AST CTO and VP of Customer Service Steven Ng recently connected with Cleanroom Technology for an extended discussion on AST’s new isolator solution. Steven gives an inside look at AST’s engineering approach, the key collaborations with Germfree and CURIS System, and considers how the numerous advancements integrated into this new barrier technology will help move…
The GENiSYS® C: Precision Automation, Intuitive Simplicity
For pharmaceutical manufacturers, every endeavor into drug development comes with a measure of uncertainty and unpredictability. It’s important to have a plan in place for when the need for adaptability arises, and that leaves many looking into proven ways to incorporate flexibility into their operational strategy. Additionally, trends in aseptic fill-finish manufacturing show customers are…
4 Ways the GENiSYS® R Sets the Standard in Aseptic Manufacturing Flexibility
Pharmaceutical manufacturing continues to see growth in the field of highly targeted, high-value liquid pharmaceuticals. Increasingly, entities like CDMOs, are looking at the most effective ways to implement adaptable, streamlined multi-modal operations designed for uptime and time-to-market optimization. In the initial phases of planning your aseptic fill-finish operation, there are many decisions to make both…
AST Live: The Engineers Behind the AST-Germfree Collaboration
Meet two of the innovative minds that helped lead the AST-Germfree collaboration on next-generation barrier technology: Engineers Michael Moore (AST) and Paula Rizo (Germfree) connected at Interphex to discuss what sets this isolator solution apart and what the journey over the course of this collaboration has been like for the two teams. https://www.youtube.com/watch?v=LEVaFl3GuzM&t=40s At Interphex 2024, AST was thrilled…
Gloveless or Less Gloves? Regulatory Trends and Automated Solutions
The following article is part 2 of a blog series that has been adapted from AST’s VP of Sales and Marketing Josh Russell’s recent article in Cleanroom Technology. In part 1, we discussed considerations for isolator configurations and AST’s approach to integrating automation in our new barrier technology solution. The most important questions to consider…
Gloveless or Less Gloves? A Real-World Approach to Optimizing Fill-Finish Automation
The following 2-part blog series has been adapted from AST’s VP of Sales and Marketing Josh Russell’s recent article in Cleanroom Technology.
Live from New York: Debuting a 100 % AST Turnkey Solution
The AST team recently returned from New York City, the site of Interphex 2024, and to say we came back motivated would be an understatement. The show was teeming with enthusiasm as life sciences professionals from across the globe gathered in the Big Apple for the premier pharmaceutical and biotechnology event. The stage was set…
Isolator Air Handling: Advancements and Considerations for Fill-Finish Processing
In the fill-finish manufacturing of parenteral liquid pharmaceuticals, maintaining product sterility is paramount. The choice of what kind of barrier technology to utilize becomes key, as operations must account for the production environment and the level of control and efficiency needed to hit key production milestones. For an increasing amount of industry professionals, isolators are…
The Quest for Sterility Assurance: A New Approach to Isolator Decontamination
One of the more challenging, essential aspects of planning for an isolated fill-finish operation is determining the approach to decontamination. Sterility assurance is a top priority, and ensuring a selected method meets regulatory thresholds is vital.
American-Made, Worldwide Benefit: Raising the Bar in Isolated Fill-Finish Manufacturing
When AST produced its first fill-finish solution in 2006, two key convictions drove that early innovation: A commitment to imagining a never-before-seen aseptic multi-format processing system that was versatile and flexible, and a desire to produce robust, American-made aseptic fill-finish products. Ahead of the release of our new isolator, we wanted to explore our commitment…
CURIS System and AST Unite to Redefine Aseptic Fill-Finish Processing Technology
**For Immediate Release** Oviedo, Florida, and Tacoma, Washington, February 5, 2024 – CURIS System, an innovator in advanced decontamination technology, and AST, a leader in aseptic fill-finish processing technology, today announced a groundbreaking partnership. This collaboration marks a significant step forward in the pharmaceutical manufacturing industry, as the two companies combine technology to revolutionize the…
The Next-Generation Isolator: A User-Friendly Solution for Fill-Finish Professionals
When AST came to the table on the design for our new fill-finish isolator, one of the first areas AST engineers wanted to address was the usability and accessibility of the isolator and corresponding operations. With our customers’ point of view in mind, we wanted to address specific points of friction routinely encountered by operators…
AST Perspectives: Reimagining Fill-Finish Isolator Design
By Steven Ng, CTO & VP of Customer Service This past October, AST announced a groundbreaking collaboration with Germfree on a new fill-finish isolator. This exciting announcement was the result of just over a year of brainstorming, research, and development, as our team at AST set out to create an isolator solution that would meet…
From Startup to Standout: Aseptic Processing Solutions for Life Science Innovators
The path to a successful life science startup is exciting, difficult, and unpredictable. Recent years have seen a volatile pattern emerge of challenge, change and innovation within the life sciences. The trend towards innovative pharmaceutical solutions is projected to continue into 2024, as breakthroughs in treatment modalities, supply chain management and patient-centered medicine are tracking…
Toolless Change Parts: A Flexible Solution
Aseptic fill-finish professionals are continually looking to augment their approach to a market that continues to grow and reflect innovations happening around sterile parenteral treatments. When considering the flexibility of an operation, it’s important to investigate every aspect of the fill-finish process. Flexibility applies to any process or technical optimization that quickens scalability and removes…
AST at Virtual Pharma Expo 2023
Josh Russell gives a live demonstration of AST’s isolated GENiSYS C as part of Virtual Pharma Expo 2023. https://www.youtube.com/watch?v=mWzKw8Mt0cU
Point of Contact: The Importance of an Intuitive HMI
As aseptic fill-finish technology continues to make strides towards broader use of robotics and automation, the usability and performance of a system’s Human Machine Interface (HMI) becomes vital to the operator. Considering the amount of complexity native to aseptic processing and the variables possible in any given system, including format, finishing, and barrier options, fill-finish…
AST Perspectives: Looking Towards a Fill-Finish Future Rooted in American Ingenuity
A Word from AST President & CEO Joe Hoff It’s common practice at AST to revisit our Mission Statement as we go about our work to provide quality fill-finish solutions to our customers. That mission—to enhance the reliability, productivity and safety of aseptic processing by offering intelligent, intuitive, and innovative solutions—informs every aspect of what…
Ready and Sealed: The RTU Advantage for Optimal Vial Sealing
For small-batch, high-value drug products, the aseptic advantages of pre-sterilized ready-to-use vials are clear. To realize the full scope of benefits that RTU vials can provide, integration with traditional processes and equipment must be carefully considered. Modern and flexible technologies such as robotics are key to seamlessly integrating RTU vials into aseptic fill/finish processes. The…
Technology Transfer Strategies: Fill-Finish Automation and Robotics
Throughout the lifecycle of a drug product, many critical target points for optimization make all the difference in the time-to-market equation. A holistic approach to the drug development process that’s able to anticipate challenges in production, regulatory requirements, and manufacturing practices should be considered. A key component of this approach is establishing early and clear…
Preparing for the Small Batch Revolution: Identifying Successful Biopharmaceutical Partnerships
Small-batch manufacturing of liquid pharmaceuticals is slated for exponential growth in the coming years and represents exciting possibilities for the future of medicine and personalized healthcare. Whether biologics, biosimilars, or orphan drugs developed in the fight against rare diseases, technology is providing solutions for the manufacturing challenges traditionally associated with small-batch aseptic processing and consequently…
Germfree Laboratories and AST Unite to Redefine Aseptic Fill-Finish Processing Technology
Revolutionary Collaboration to Introduce an American-Made Turn-Key Solution with Unparalleled Innovation Ormond Beach, Florida, and Tacoma, Washington, 09-October-2023 — Germfree Laboratories, a global leader in isolator technology for aseptic processing and containment in the pharmaceutical sector, and AST, a pioneer in aseptic fill-finish processing technology, are thrilled to announce a revolutionary partnership. This landmark collaboration…
Fill-Finish for Biosimilars: Ready, Set, Develop!
The last four years have seen remarkable developments in the field of cell and gene therapy. In 2019, the FDA forecasted that the US is likely to add 10-20 cell and gene therapies per year until 2025, and current prognostication has development tracking at that speed.
503 Pharmacies: Integral Partners in the Healthcare Industry
The last four years have seen remarkable developments in the field of cell and gene therapy. In 2019, the FDA forecasted that the US is likely to add 10-20 cell and gene therapies per year until 2025, and current prognostication has development tracking at that speed.
Cell and Gene Therapy: 2023 and Beyond
The last four years have seen remarkable developments in the field of cell and gene therapy. In 2019, the FDA forecasted that the US is likely to add 10-20 cell and gene therapies per year until 2025, and current prognostication has development tracking at that speed.
The Future of CDMOs: Aseptic Solutions for Growth and Innovation
When deciding on an aseptic fill/finish machine, the choice to use a gloved or gloveless isolator will arise – and we want to help with that decision. There are a few attributes to consider when choosing between a gloved or a gloveless isolator.
Gloved vs. Gloveless Aseptic Filling Machines
When deciding on an aseptic fill/finish machine, the choice to use a gloved or gloveless isolator will arise – and we want to help with that decision. There are a few attributes to consider when choosing between a gloved or a gloveless isolator.
4 Factors to Consider when Scaling Up
Moving forward to the next phases of clinical trials is an extremely exciting and hectic time. Understanding how scaling up affects your aseptic filling operations is essential. Here are 4 topics to consider that will help ensure that your drug product will be taken care of and be ready for production while increasing your ROI.
Using Robotics in the ATMP Space
The use of robotics to manufacture Advanced Therapy and Medicinal Products (ATMP) solves many challenges, including significantly limiting exposure to human generated contamination. There are steps to mitigate contamination points but with the strong endorsements recently made within the current revision to Annex I encouraging the use of robotics (Section 8.9) 1, eliminating human interventions should be considered seriously.
7 Things to Know When Evaluating Small-Scale Aseptic Filling Technology
Small-scale aseptic filling presents its own unique set of challenges. There are questions like “how fast do you need your machines to run?” or “What kind of material do you need to process?” Those types of questions can lead to other ones that possibly have more complicated answers. To help, we’ve broken down 7 things…
Challenges Facing Aseptic Manufacturing in the Cell and Gene Therapy Market
Cell and gene therapies continue to gain significant traction in the biopharmaceutical world. While this technology continues to grow, challenges will inevitably follow. When it comes to aseptic fill/finish challenges, these 4 are ones to consider:
Quick Delivery Program Supports Critically Ill Patients
The reason for the existence of the pharmaceutical world is simple – People need medicine. How you get that medicine to the patient is the big question. While many different facets of medicine were brought to light by the COVID-19 pandemic, possibly none were viewed as more important than the need for increased urgency when…
AST Introduces New Customer Care Team Members
AST is pleased to introduce you to two new members of our Customer Care team. Matt Taylor (right) will serve as Customer Care Manager, and Antonio Bobbitt (middle) joins AST as Project Coordinator. In addition, after 2 years on AST’s controls technician team, Thomas Beebe (left) has been promoted to Customer Care Engineer. Matt Taylor…
AST’s New Business Development Team Members
AST is excited to introduce you to our newest members of the company!
AST Announces New Vice President of Sales and Marketing
We are excited to announce the addition of Josh Russell as the Vice President of Sales & Marketing at AST.
AST Announces Availability of Quick-Delivery Isolated Fill-Finish Equipment
AST is excited to announce the availability of quick-delivery of the most popular isolated equipment within 6 months of purchase.
Providing Flexible Aseptic Solutions: The AST Way
Download our case study to learn more about AST’s latest project with Catalent.
Considerations for Pre-filled Syringes for 503(b) Compounding Facilities
AST, a leading aseptic fill- and finishing machine manufacturer, and SCHOTT, a global expert in drug containment solutions, share their expertise on how to help 503(b) compounding facilities bring acute care drugs to market quicker, provide a safer drug administration process, and minimize healthcare and hospital costs.
AST at CPhI North America
AST’s Keith Dodson and Chaise DeVries will be at the CPhI North America live event next week, August 10-12. To schedule a meeting, reach out on LinkedIn or contact us. We hope to see you there!
Reinventing your Aseptic Processing
Today, new drug products and multi-product facilities demand higher precision and flexibility than ever before.
Use of Robotics in Aseptic Fill Finish
AST and ISPE India are proud to present “Use of Robotics in Aseptic Fill Finish,” a webinar hosted by Joe Hoff and Keith Dodson
AST Partnership with Quality Chemical Laboratories
We are proud to announce our partnership with QCL on both a GENiSYS® C and GENiSYS® R
Aseptic IPS Technologies Tour
Joe Hoff joins industry experts in IPS’s annual technology tour for INTERPHEX 2020
Improve Sterility Assurance And Data Integrity 503B Compounding
Sheila Dell, Ph.D, discusses automated technology and how it can improve sterility assurance and data integrity in the compounding sector.
Overcome the Challenges of Small Batch Production
AST’s Matthew Gorton offers insights on solutions to common problems in small batch production
Goodwin Biotechnology Partnership
AST and Goodwin Biotechnology Partner on a GENiSYS® C20 Aseptic Small Batch Filling & Closing Machine
ISPE Europe 2020 Virtual Booth
Visit our virtual booth for the ISPE Europe Annual Conference
AST Technology Tour
Join AST’s Chairman and CEO, Joe Hoff, on September 16th for this INTERPHEX and IPS Technology Tour.
The Advantages Of Robotics In Aseptic Fill Finish
AST’s latest whitepaper on robotics, now available for download.
The Robotic Revolution
A look back at the recent history of aseptic fill/finish and how AST brought new multi-format flexibility to the market with the development of the first ASEPTiCELL®
AST at Compounding Pharmacy Compliance’s 2020 Digital Week
AST VP of Business Development Matthew Gorton provides an analysis of the issues that are driving compounding facilities to invest in automation for the audience of CPC Digital Week
The Advantages Of Robotics In Aseptic Fill Finish
AST’s latest whitepaper on robotics, published in full on the Pharmaceutical Online website
How Robotics Offer New Answers to Today’s Challenges
Staubli presents a panel discussion webinar on robotics with Joe Hoff
AST Statement Regarding COVID-19
The following statement from president and CEO Joe Hoff explains AST’s commitment to maintaining the utmost safety standards while remaining fully operational throughout the current COVID-19 outbreak.
Sterile Filling Challenges for Early-Phase Product Development
Joint webinar with Pii on aseptic product development
AST Upcoming Events
We are excited to meet you at several upcoming industry events around the globe. Continue reading below for information on where to find us at Pharmapack and the ISPE Aseptic Conference!
November 2019 Events
We are very excited to announce our presence at a number of upcoming pharmaceutical events around the globe. Click the link below for information on where to find us at CPhI Worldwide 2019, AAPS in San Antonio, and CPhI India 2019! CPhI Worldwide 2019 AST is excited to exhibit at CPhI Worldwide for the first…
Bridging the Divide – Streamline The Process Development & Tech Transfer Process
Preparing drug products for tech transfer can be a daunting task. Traditional process development practices tend to be labor intensive, difficult to scale to a manufacturing environment, and gathering the critical process data to make informed decisions can be a challenge. This article describes how the advantages of AST’s GENiSYS® system can streamline the tech…
Delivering Flexibility Without Complexity
The pharmaceutical and biotechnology products currently under development or being commercialized are specific and personalized in nature. Given this targeted nature the value of these products are high and batch sizes are small. This brings new manufacturing challenges to aseptic fill-finish operations where traditional approaches are not meeting the flexibility, yield or efficiency these products…
Pii and AST Partner On GENiSYS R Aseptic Filling and Closing Machine
Pii and AST Partner On GENiSYS R Aseptic Small Batch Filling & Closing Machine HUNT VALLEY, MD and TACOMA, WA (April 2, 2019) – Pharmaceutics International, Inc. (Pii), a Contract Development and Manufacturing Organization (CDMO) and Automated Systems of Tacoma, LLC (AST), a leading provider of flexible aseptic filling systems, announce their partnership on the…
AST to Unveil GENiSYS R Robotic Filling System at Interphex
AST will be unveiling its latest multi-format robotic filling system, the GENiSYS R, at Interphex April 2nd-4th. The GENiSYS R is our newest system innovation designed specifically with cell, gene and regenerative applications in mind. Stop by Booth #3853 to experience this latest in AST’s advanced aseptic systems and speak with our product experts to…
AST at the ISPE Aseptic Conference
Interested in learning more about current and future trends in aseptic manufacturing? The 2019 ISPE Aseptic Conference on March 18th and 19th in Bethesda, MD is a must attend conference. This year’s conference will be touching on industry hot-button topics such as: aseptic robotics in cleanrooms, Annex I updates, product development strategies, disposable technologies, and…
2019 PDA Annual Meeting
AST is excited to be joining industry experts at the 2019 PDA Annual Meeting happening March 11th- 14th in San Diego, CA. This year’s conference theme is Solving Manufacturing and Supply Challenges for Current and Future Medicinal Products and will cover wide-ranging topics focusing on improving existing processes and new technologies that can be leveraged…
AST Presents At ISPE Facilities of the Future Conference
The 2019 ISPE Facility of the Future Conference will explore what pharmaceutical manufacturing facilities will look like in the future and will feature presentations from various industry thought leaders and experts sharing current projects highlighting what these “facilities of the future” look like. New manufacturing challenges, technologies and regulator factors impact the ability of today’s…
AST Launches GENiSYS® R Robotic Filling and Closing Machine
AST, a leading innovator of advanced aseptic filling and closing systems developed for the pharmaceutical and biopharmaceutical industries, recently announced the release of its new GENiSYS® R product line for aseptic filling and closing of nested ready-to-fill vials, syringes and cartridges. TACOMA, WA – AST, a leading innovator of advanced aseptic filling and closing systems…
Tacoma PharmaSolutions Private Limited
AST announces Indian subsidiary, Tacoma PharmaSolutions Private Limited – a wholly owned Indian subsidiary to keep pace with the regions growing bio-similar and biologics manufacturing industries. In a move to expand its footprint in India, Automated Systems of Tacoma, LLC (AST), a leading aseptic fill/finish equipment manufacturer, is pleased to announce the establishment of a…
CPhI – PMEC North America
AST will be participating in the first ever P-MEC machinery zone at the CPhI North America 2018 event in Philadelphia April 24th-26th. AST will be showcasing our GENiSYS c10 filling and closing machine that is quickly becoming the relied upon solution for small batch clinical, commercial drug product and development applications. Come visit us at…
Interphex 2018
AST is excited and ready to again be participating in one of the leading pharmaceutical industry technology events Interphex 2018 at the Javits Center in New York City April 17-19th. AST will be showcasing our GENiSYS combi-machine and several of our product experts will be at the show fielding questions about our latest innovations like…
AST at PDA’s 2018 Annual Meeting
AST is joining industry thought leaders and experts at the 2018 Parenteral Drug Association (PDA) Annual Meeting in Orlando, Florida March 19th -21st. This year’s conference theme is Agile Manufacturing Strategies: Driving Change to Meet Evolving Needs and will discuss emerging technologies and latest trends being used to provide patient focus medicines. In addition to…
Facilities of the Future Conference
Interested in learning what thought leaders are envisioning for the future of manufacturing for pharmaceuticals, biopharmaceuticals and personalized gene and cell therapies? If so, the ISPE Facilities of the Future Conference is a must attend conference. ISPE’s Facility of the Future Conference is February 20th-22nd in Bethesda, MD. AST is excited to be participating and…
ISPE 2018 Aseptic Conference
AST is excited to be participating and exhibiting at this year’s ISPE Aseptic Conference March 6th & 7th in Ruston, VA. Josh Russell, AST’s Director of Business Development, will be participating in an industry forum discussing robotics and their application in aseptic processing applications. AST will also be exhibiting at booth #409. Please stop by…
Robotics & Isolator-Barrier Systems: The Perfect Combination for Advanced Aseptic Processing
The aim of advanced aseptic processing is the elimination and absolute control of all sources of contaminations – most importantly, human generated contamination. Robotics and isolator-barrier systems will be the core technologies in meeting this endeavor. The Parenteral Drug Association (PDA) describes an Aseptic Process as, “The process for manufacturing sterile products by which microbiological…
Successful CPhI-PMEC India Premiere
This year’s CPhI-PMEC India show was a success by all measures. With the GENiSYS Lab Container Filling System and Vial Sealing System on display attendee’s were able to see the systems in operation and gain a better understanding of the key features and benefits of these solutions in helping them overcome some of the common…
ASHP Midyear Meeting 2017
AST is excited to be participating at this years American Society of Health-System Pharmacists (ASHP) Midyear meeting Decmber 3rd -7th in Orlando, FL. The ASHP Midyear Cinical Meeting is the largest gathering of pharmacists in the world. With its focus on improving patient care, the meeting is a dynamic venue discussing important issues relevant to…
Tired of Hand Filling? AST’s Benchtop Systems Could be Your Answer
Aseptically processing small batches of sterile drug products has many unique challenges that have to balance cost, flexibility and quality. Normally for small batches companies default to manually filling and processing approaches with very little automation. Unfortunately, given the intimate interaction manual processing has with these products, contamination risk and process variability can be high.…
AST Launches New Line of Benchtop Machines for Aseptic Processing
AST launches a new line of benchtop machines specific to meeting the needs of small batch aseptic applications. The product family includes a Container Filling System, Container Closing System and Vial Sealing System that together can be used to fill, close and seal vials, syringes and cartridges. These benchtop systems have been designed to include…
2017 ISPE Biopharmaceutical Manufacturing Conference
AST is excited to again be partcipating at the International Society of Pharmaceutical Engineers (ISPE) Biomanufacturing Conference. This years conference will be held in San Francisco, CA., December 4th-6th. This year’s theme Launch Readiness: Operational Agility for Today’s Challenges, has educational tracks that highlight various strategies that are being by biopharma companies to shorten launch…
Processing Ready-To-Use Vials with Proven Vial Capping Solutions
Pre-sterilized, Ready-To-Use (RTU) vials are quickly gaining adoption within the biopharma industry, particularly for small batch applications. RTU vials simplify the processing requirements and facilities necessary to aseptically fill/finish drug products by eliminating equipment, utilities and production space required for container preparation. To realize the full breadth of benefits RTU vials can provide, integration with…
CPhI / P-MEC India 2017
AST will be exhibiting at the CPhI / P-MEC Pharmaceutical Equipment & Technology Exposition in Mumbai, India November 27th – 29th. AST is excited to be exhibiting for the first time at the P-MEC India Expo, and to unveil and showcase our latest aseptic equipment innovations at this event. Come visit us at Booth #AC31,…
2017 ISPE Annual Meeting
AST is pleased to be continuing our long standing support of the International Society of Pharmaceutical Engineers (ISPE) and will be attending its 2017 Annual Meeting October 29th – November 1st in San Diego, California USA. This year’s annual meeting themed: Driving Innovation to Advance Patient Therapies is focused on bringing to together industry thought…
2017 PDA Annual Meeting & Exhibition
AST is joining industry professionals and experts at the 2017 Parenteral Drug Association (PDA) Annual Meeting in Anaheim, California April 3rd – 5th. Come and visit AST at Booth #115 to discuss your aseptic manufacturing application and learn more about how our innovated production options can be leveraged to solve your important aseptic challenges. Tacoma,…
Interphex 2017
AST will be exhibiting at the Interphex 2017 Pharmaceutical Equipment & Technology Exposition at the Javits Center in New York City, March 21st – 23rd. AST will be showcasing our newest GENiSYS small scale filling machine and hosting a VR Tour of our latest ASEPTiCell robotic isolated filling system at the 2017 Interphex Exhibition. Come…