PRODUCTS
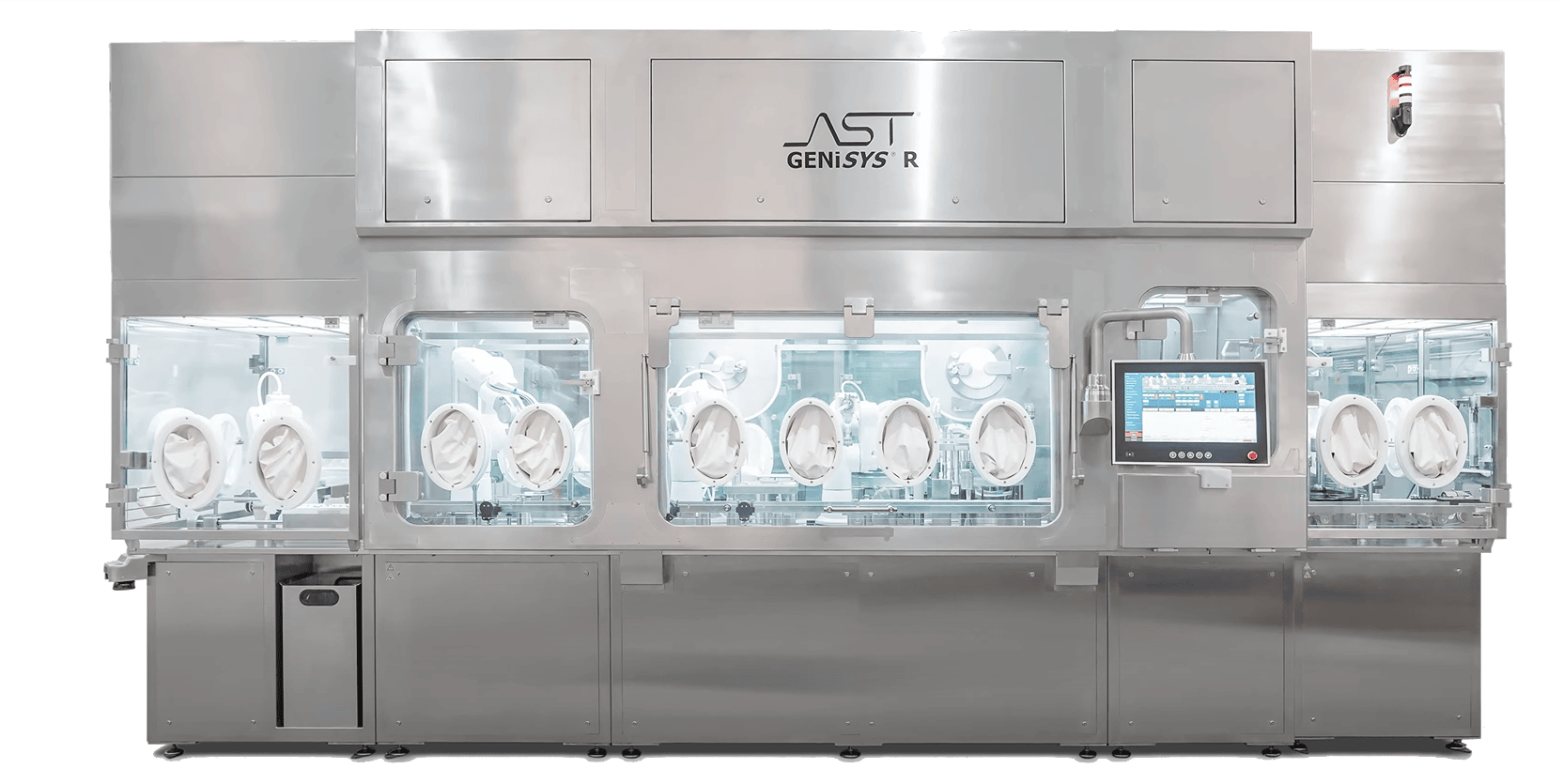
The GENiSYS® R: Premier Robotics and Automation for Highly Targeted Aseptic Processing
In the Know
Capabilities
- Proven and standardized modules configurable to the full scope of drug development
- Universal RTU container processing capabilities
- cGMP technology across all modules
- Scalable solutions for easy validation and efficient technology transfer
- Product stewardship measures for quality control and high-yield operations
- Full ramp-up support including qualification services and full documentation packages
- Responsive, 24/7 aftermarket support throughout the life of your equipment
- Processes up to 20 units per minute
- Toolless change parts
- Processes vials, syringes, and cartridges on the same line with minimal changeover time between formats
- Peristaltic and rotary piston pump options
- Configurable, recipe-driven production
- Centralized HMI for operation and data retention, and optional EBR system for 21 CFR Part 11 Compliance
- 24/7 runtime capability for uninterrupted batch processing
The GENiSYS® R utilizes precisely designed robotics and automation in a quality-by-design approach specifically designed for the demands and specifications of sterile injectable products. Features include:
- Low-risk, aseptic environments (ISO 5 – grade A)
- FDA compliant
- Non-absorptive & microbially resistant
- VPHP compatible
- Reduced human interventions
- Intuitive design with minimal points of failure
- Gentle container handling
- Specialized format handling, including West CZ Tray packs
Lead time and machine changeover requirements are crucial to the time-to-market equation for pharmaceutical manufacturers. The built-in philosophy at AST approaches fill-line configurations in an adaptable way, able to meet the customer’s specifications. Like grabbing the right tools for the job from a toolbox, our modular approach enables AST engineers to capture the majority of requirements with our existing modular solutions, which allows very rapid configuration, delivery, validation, and launch of an operation.
The available modules on the GENiSYS® R are:
- SABO – Semi-Automatic Bag Opening
- ABO – Automatic Bag Opening
- MTO – Manual Tub Opening
- ATO – Automatic Tub Opening
- FCM – Fill / Close Module
- LPM – Lyophilization Prep / De-Nesting Module
- VSM – Vial Sealing Module (also available in a linear configuration)

Interactive Diagram of GENiSYS® R | Click a Hotspot
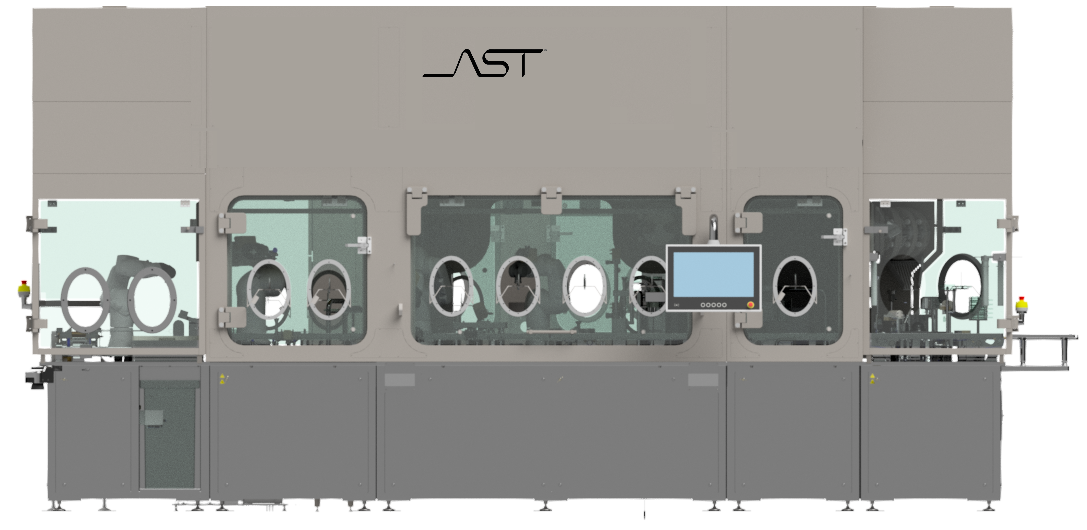
Automated Bag Opening with hygienic trash removal and hands-free transfer
Robotic tub handling with seal removal and staging with minimal particle
generation
Recipe-driven filling and closing of containers with in-line quality controls and reject sorting
You've seen the GENiSYS® R in motion...
Perhaps it's time to discover how it would work with your application?
Meeting Industry Needs | Applications
CDMOs and CMOs are crucial partners to biotechnology and life science companies, offering end-to-end service and development for the latest liquid pharmaceuticals. More than ever, contract manufacturers are looking for flexible, scalable platform technology. The GENiSYS® R has been a resource for many looking for the best in multi-modal fill-finish technology.


With proven, validated key technologies like pumps, decontamination methods, IPC protocols, and filling and closing methods able to transfer seamlessly from one state of drug development to the next, the need for revalidation is reduced, and valuable time is saved over the life of an operation. AST’s automated approach allows for uncorrupted data traceability across the entire drug development progression, crucial for maintaining cGMP compliance and preserving process knowledge for successful technology transfers.
Use Cases

A leading global CDMO looking to expand its cell and gene production portfolio partnered with AST on an innovative GENiSYS R system for multi-modal production.
Download the Case Study


