Bench-Top Vial, Syringe & Cartridge Filling System
Bench-Top Vial, Syringe & Cartridge Filling Machine
AST’s Container Filling System (CFS) is a bench-top, semi-automatic machine used for the filling of pre-filled syringes and cartridges, and the filling and stoppering of vials and bottles. The system is designed to meet the demanding requirements for small batch processing of sterile injectable products.
The Container Filling System was designed with cGMP in mind. Its compact, aseptic design provides complete compatibility with cleanrooms, bio-safety cabinets, laminar airflow hoods, and aseptic isolator environments.
The system’s versatility, features, and compact size make it ideally suited for labs and cGMP small batch filling applications.
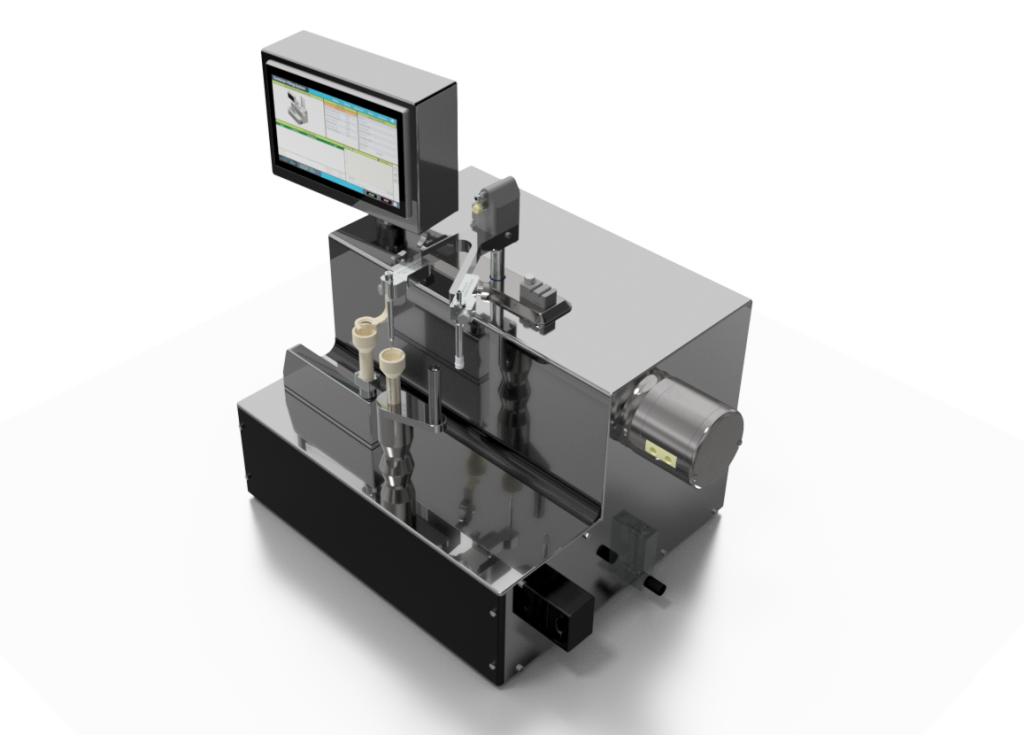
Consistent & Accurate Container Filling
The Container Filling System is integrated with an advanced product dispense system that provides consistent and accurate product dispensing. The integrated peristaltic pump provides accurate dispensing with minimal product shear while using a pre-sterilized single-use fluid path. When integrated with an electronic weigh scale, the system can fill the container while measuring the dispensed material and providing automatic fill adjustments to the pump as required. The system’s electronically controlled axis inserts the filling needle into the container and slowly lifts to minimize shear, foaming or product agitation.
Bench-Top Container Filling Process
The empty container is manually placed on the support at the center of the machine. For vial filling, a serum or lyo stopper is placed on a support stand immediately adjacent to the container support. Once positioned, the system will automatically transfer the container to the electronic weigh scale and record the tare weight of the container. With the container on the balance, the filling needle will go into the container and dispense the product as it slowly lifts from the inside of the container. Once the filling cycle is complete, the final fill volume is recorded and any fill adjustments are automatically performed. The system will transfer the container back to the center support. For vials, the system will pick and place the previously placed stopper onto the vial and the operator will manually remove it from the machine. Syringes and cartridges will be removed manually and closed on the Container Closing System (CCS).
Capabilities
The Container Filling System is integrated with advanced system features to provide consistent and accurate product dispensing. The system is integrated with a peristaltic pump that provides accurate dispensing with minimal product shear. When integrated with a weight scale the system can fill the container to measure the dispensed material in real-time, and provide automatic adjustment to the pump. The system’s electronically controlled axis inserts the filling needle to the container and slowly lifts to minimize shear, foaming or product agitation. For applications where a peristaltic pump is not ideal, such as dispensing viscous materials, the system can be configured with a rotary piston pump.
The Container Filling System provides the operator with complete control of the critical filling process. Using the HMI touchscreen the operator is able to create recipes that control parameters such as; dispense volume, filling needle depth into the container, fill needle retraction speed, pump speed, and many other variables. All the critical process variables can be monitored and recorded with the optional Electronic Batch Reporting (EBR) system for batch documentation, process analysis, technology transfer, and optimization.
- ASTView high-resolution interface provides intelligent and intuitive control of the system for creating unique recipes and real-time process monitoring
- Dispense system provides accurate and repeatable container filling with automatic volumetric monitoring and adjustment
- Process flexibility allows the system to fill a wide array of containers with materials of various sensitivity and viscosity ranges
- Electronic Batch Report (EBR) System records critical process information that can be used to create a 21 CFR Part 11 compliant batch report to simplify the technology transfer or regulatory filing processes
- Compact design makes the system ideally suited for aseptic isolators, biological safety cabinets or laminar airflow hood
- R&D, preclinical and process development
- Clinical trial materials manufacturing
- Gene & cell therapies
- Small batch commercial drug products
- Stability studies
- Compounding pharmacies
Benefits
- Accurate and gentle product dispensing
- Fill vials, syringes and cartridges with a single machine
- Optional integrated weigh scale for real-time fill weight measurement
- Can be configured with peristaltic or rotary piston pumps
- Intuitive touchscreen interface for easy system operation
- Inert gas purging during filling
- Tool-less format change parts
- Optional Electronic Batch Reporting (EBR) system
- Compact system footprint allows placement within an isolator, bio-safety cabinet or laminar airflow hood
Ready to get started?
Request a consultation to get started
Technical Information
| System Dimensions (LxWxH) | 840mm x 500mm x 800mm [33" x 20" x 32"] |
| Containers | Vials, Syringes & Cartridges |
| Vial Finish | 13mm, 20mm, 32mm |
| Vials Sizes (Dia./Height) | Ø12mm-47mm / 35mm-100mm |
| Syringe Sizes | 0.5mL to 50mL |
| Cartridge Sizes | 1mL to 20mL |
| Other Container Options | Tooling for non-standard/custom container formats |
| Fill Accuracy | Up to ±0.5% |
| Materials of Construction | Pharmaceutical grade stainless steel, plastics, and elastomers |
| Human-Machine Interface (HMI) | ASTView on a 10" color touchscreen |
| Pump Options | Peristaltic & Rotary Piston |
| Available Options | In-process fill weight verification (IPC) Pre- and post-fill container closing with inert gas Single-use disposable fluid paths Product bag support stand Isolator compatible system upgrade Electronic Batch Report (EBR) System |

