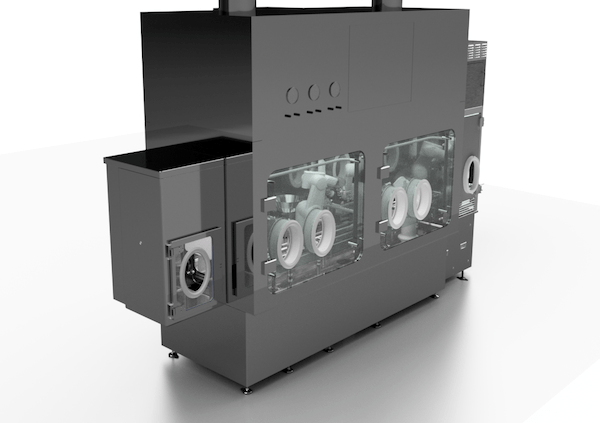
The aim of advanced aseptic processing is the elimination and absolute control of all sources of contaminations – most importantly, human generated contamination. Robotics and isolator-barrier systems will be the core technologies in meeting this endeavor.
The Parenteral Drug Association (PDA) describes an Aseptic Process as, “The process for manufacturing sterile products by which microbiological contamination is eliminated from the product and product contact surfaces protecting the product from sources of contamination” (PDA Technical Report No. 44, 2008). The challenge for pharmaceutical drug manufacturers is to ensure that the products they manufacture are made in a manner that precludes microbiological contamination. This is especially important for injectable or parenteral drugs, which are the products having the highest risk, because the injection bypasses all barriers nature has provided for the patient. One of the greatest sources of contamination risks are human borne contamination from operators in and around aseptic production. Therefore, to isolator the human contamination using aseptic barrier systems and eliminating the need for aseptic intervention is paramount.
Advanced Aseptic Processing (AAP) is the utilization of automated technologies such as robotics and physical barriers in order to eliminate operator intervention with the process, open product containers, and exposed product contact surfaces. The key to AAP operation is maintaining absolute control of contamination sources through physical and aerodynamic means against contaminant migration into the sterile environment.
Isolators & Restricted Access Barrier Systems (RABS)
Isolators and Restricted Access Barrier Systems (RABS) are rigid wall mini-environments that provide a physical and aerodynamic barrier between the operator and the sterile drug manufacturing process enclosed within the interior environment. Both isolators and RABS, when operated properly, will provide an ISO 5 cleanroom environment meeting the regulatory requirements for particulate and microbiological concentrations. Although the internal requirements are the same for RABS and isolators, there are several key design features that differentiate the two systems.
Isolator Attributes:
- Totally enclosed and sealed stainless steel shell glove box type system
- HEPA filtered unidirectional air within the enclosed aseptic interior
- Positive or negative pressure relative to the background cleanroom environment
- In-situ bio-decontamination most commonly with hydrogen peroxide vapor
- The use of Rapid Transfer Ports to bring materials into and out of the isolator chamber
- Access and manipulation from outside of the isolator is performed using a half-suit or glove ports
RABS Attributes:
- Physically enclosed barrier around the critical aseptic zones
- HEPA filtered unidirectional air within the enclosed aseptic interior (commonly referred to as an “active” RABS)
- Supplied HEPA filtered air is exhausted into the background cleanroom
- Slightly positive pressure relative to the background cleanroom
- Manual high level bio-decontamination of the aseptic zones using a sporocidial reagent and isopropyl alcohol (IPA)
- The use of Rapid Transfer Ports to bring materials into and out of the isolator chamber
- Access and manipulation from outside of the isolator is performed using a half-suit or glove ports
Robotics & Automation in Aseptic Manufacturing
Aseptic manufacturing in general is a highly repetitive activity that requires a high degree of reproducibility in order to create a high-quality product. Robots are the ideal platform to provide the highly accurate and repeatable operation demanded by aseptic processing. Additionally, robots can operate in environments where humans cannot. This becomes particularly important in applications that require containment of highly active and potent compounds. Robots also can be safely integrated into critical aseptic areas, because they generate extremely low non-viable and viable particulate levels having compatibility with ISO 5 environments.
Isolated robotics in aseptic manufacturing has one particular advantage over traditional aseptic machinery; flexibility. Robots are completely adaptable and can change with the product or process. If the current application or the container format has changed, the robot system can be reprogrammed for another manufacturing process with minimal investment. To adapt the robot to another application it would require new or modified end of arm tooling, processing fixtures, programming, and possibly some additional utilities like vacuum and sterile air. The turnaround time and resources required to reconfigure a robot is considerably less than the investment in a new, dedicated machine or filling line.
Summary
They key to Advanced Aseptic Processing is the elimination and absolute control of all sources of contaminants, most importantly human generated contamination. Robotics and isolator-barrier systems will be the core technologies that further this initiative. A properly integrated robot system that is compliant to ANSI/RIA safety requirements combined with a properly designed and implemented isolator-barrier system offers a flexible robotic cell that is compatible with the strictest of regulatory standards. Ultimately, the many layers of operator and product protection provided by isolated robotics offer superior control over ingress of contamination when compared to traditional cleanroom manufacturing and thereby protecting product quality and minimizing risk to patient safety.
This article is created from whitepaper excerpts written by Josh Russell with AST called Robotics and Isolator-Barrier Systems: The Perfect Combination for Advanced Aseptic Processing (Published Innovations in Pharmaceutical Technologies, 2010). For the entire article please click here.