Using Robotics in the ATMP Space
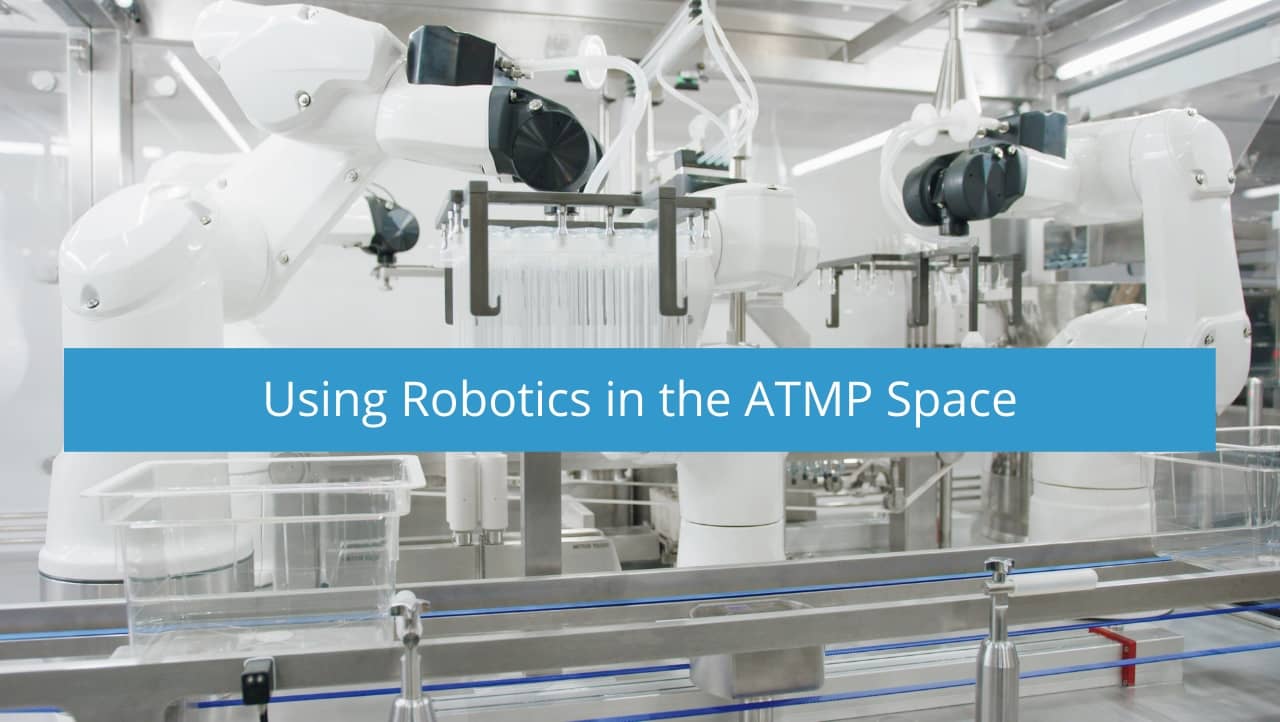
The use of robotics to manufacture Advanced Therapy and Medicinal Products (ATMP) solves many challenges, including significantly limiting exposure to human generated contamination. There are steps to mitigate contamination points but with the strong endorsements recently made within the current revision to Annex I encouraging the use of robotics (Section 8.9) 1, eliminating human interventions should be considered seriously.
The endorsements made within Annex I encourage the use of robotics and automation with isolator-barrier technology. The union of these technologies meets the regulatory goals of eliminating the most abundant source of error and contamination, the people involved within the aseptic manufacturing processes. The average human has ~1-3% of their body weight as microorganisms, outweighing human cells 10:12, and more human interaction with production processes means more microbes being shed and higher probability of contamination.
Current ATMP production methods have a high degree of exposure to human-generated contamination. This is primarily because ATMP products are made in lower quantities which typically use less automation and more manual production steps than traditional biologics and have many unique processing steps. The increase in the use of these products has led to a few small advancements in production quality, but there will need to be an increase in productivity improvements as ATMP’s become more prevalent in the medical field. Introducing robotics to the production of ATMP’s will eliminate every possible human contamination exposure point, making robotics an ideal solution for both productivity and product quality.
Supplementary good manufacturing practices (GMP) like Pharma 4.03 being worked into the manufacturing process will continue to push robotics to the forefront of aseptic manufacturing processes. Pharma 4.0 discusses a holistic control strategy with robotics, and automation along with the sensors and AI systems that drive the robotic systems will be necessary to achieve the full benefits of a holistic strategy. These systems will be interconnected within a broader network of systems that will drive the robotic systems to produce the right product at the right time. In a function where sterility is necessary by regulation, the benefits of robotic systems with isolator-barrier technology are obvious and should quickly become the standard for manufacturing platforms.
Robotics also address the challenge of product waste and making use of all the drug product available. Personalized autologous therapies are a perfect example of this. Autologous therapies use patient supplied materials, such as white blood cells or bone marrow. Since a person only has a certain amount of an autologous product available, it is critical that waste is all but eliminated during the manufacturing process. Robotics assists in minimizing product waste by handling products gently, safely, and efficiently. In the event a reject is identified, robots can remove the rejected container while leaving the others untouched.
Overall, the use of robotics in the relatively new ATMP space will greatly enhance nearly all aspects of the industry. With regulatory requirements and GMP recommendations trending towards less human interaction and more robotic automation, robotics is the way of the future for the ATMP industry. Contact our aseptic processing experts today and find out how we can help your organization.
References
- European Commission. (August 2022) The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. Retrieved from https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf
- National Institute of Health (June 2012) NIH Human Microbiome Project defines normal bacterial makeup of the body. Retrieved from https://www.nih.gov/news-events/news-releases/nih-human-microbiome-project-defines-normal-bacterial-makeup-body#:~:text=Methods%20and%20Results,vital%20role%20in%20human%20health.
- ISPE (April 2021) Holistic Control Strategy: From ICH Quality Guidelines to Pharma 4.0™. Retrieved from https://ispe.org/pharmaceutical-engineering/march-april-2021/holistic-control-strategy-ich-quality-guidelines-pharma

