Archives for paul@extractmg.com
AST, a leading provider of flexible aseptic filling systems, has announced the placement of a next-generation GENiSYS® C fill-finish system at the Ascend Advanced Therapies (Ascend) Alachua, FL facility.
Drug shortages are an ongoing reality in the pharmaceutical industry, as the seriousness and length of drug shortages have steadily increased over the past five years. Of the shortages recorded in 2023, over half were sterile injectable products. In such cases, compounding pharmacies find themselves meeting the call for production as designated 503 entities are […]
AST is excited to introduce Sean Silvey, who is joining us as Product Manager. Sean has been leading critical product development and technical communication initiatives for over a decade and has an extensive background in mechanical engineering. Sean has a passion for connecting creative problem-solving and strategic thinking with customer-centered solutions. As part of AST’s […]
When determining your approach to building an aseptic processing line, one of the first choices customers face is between custom builds to meet product specifications versus the use of modular, configurable technology.
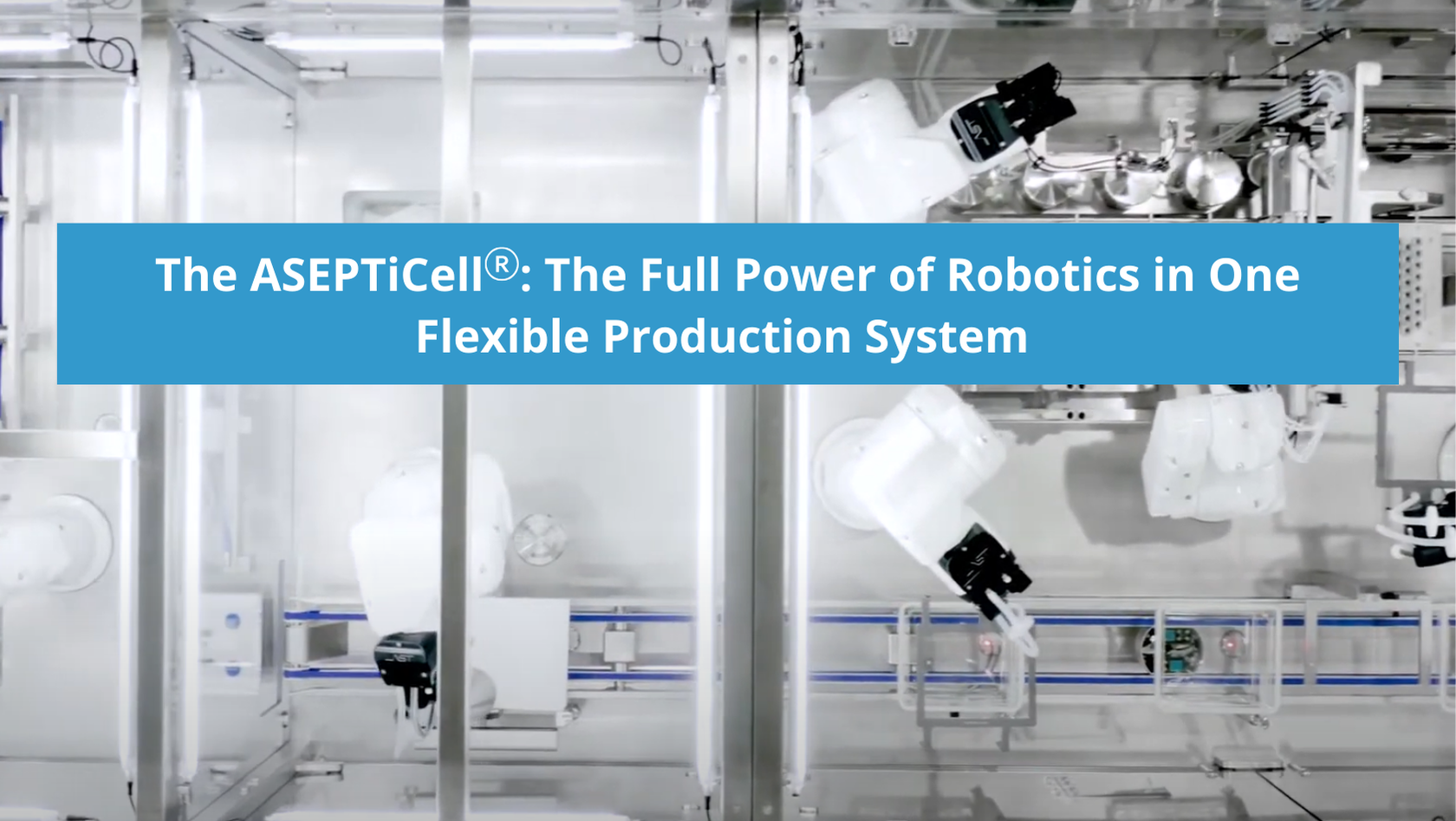
The ASEPTiCell is where the full power of AST’s automated and robotic solutions meet precision modular engineering for the commercial production of medium-batch liquid pharmaceuticals. Fully automated from start to finish, this highly intuitive, flexible fill-finish system was the industry’s first aseptic multi-format processing machine capable of processing ready-to-use nested vials, cartridges, and syringes on the same line.
Josh Russell, AST Vice President of Sales & Marketing, sat down with Pharmaceutical Technology at Interphex 2024 to discuss recent developments and advancements at AST, including an American-made isolator solution, an innovative new decontamination technology, and the benefits of AST’s comprehensive, integrated turnkey approach. Highlights and Themes: Modular technology: AST’s innovative approach to aseptic fill-finish […]
With the revised Annex 1 coming into effect last year, the pharmaceutical industry continues to strategize the best way to attain and maintain compliance with the intensive regulation on sterile medicinal products. Aseptic fill-finish manufacturing, for its part, is established on precise science and the highest standards of safety. While much of the science, technology, […]
Josh Russell, Vice President of Sales and Marketing, recently spoke to American Pharmaceutical Review on AST’s latest aseptic processing solutions. American Pharmaceutical Review, a leading journal for business and technology, connected with Josh at Interphex 2024, where AST exhibited our latest generation GENiSYS® C housed inside AST’s new isolator solution. The exhibit showcased the latest […]
Josh Russell, AST’s Vice President of Sales and Marketing, recently took part in the Virtual Pharma Expo: Aseptic Fill/Finish, Manufacturing and Packaging with a walkthrough of some of the latest AST technology, demonstrated on our GENiSYS® R robotic line.
AST’s Director of Engineering Spencer Bolte walks through some of the key design considerations on AST’s new isolator including operator-centered solutions and a groundbreaking new decontamination technology for complete 60-minute cycle times, including aeration. The newly unveiled AST isolator and the latest generation GENiSYS C are two of AST’s intuitive, compact solutions for high-value liquid […]