Cell and Gene Therapy: 2023 and Beyond

The last four years have seen remarkable developments in the field of cell and gene therapy. In 2019, the FDA forecasted that the US is likely to add 10-20 cell and gene therapies per year until 2025, and current prognostication has development tracking at that speed.
The Future of CDMOs: Aseptic Solutions for Growth and Innovation

When deciding on an aseptic fill/finish machine, the choice to use a gloved or gloveless isolator will arise – and we want to help with that decision. There are a few attributes to consider when choosing between a gloved or a gloveless isolator.
Gloved vs. Gloveless Aseptic Filling Machines

When deciding on an aseptic fill/finish machine, the choice to use a gloved or gloveless isolator will arise – and we want to help with that decision. There are a few attributes to consider when choosing between a gloved or a gloveless isolator.
4 Factors to Consider when Scaling Up

Moving forward to the next phases of clinical trials is an extremely exciting and hectic time. Understanding how scaling up affects your aseptic filling operations is essential. Here are 4 topics to consider that will help ensure that your drug product will be taken care of and be ready for production while increasing your ROI.
Using Robotics in the ATMP Space
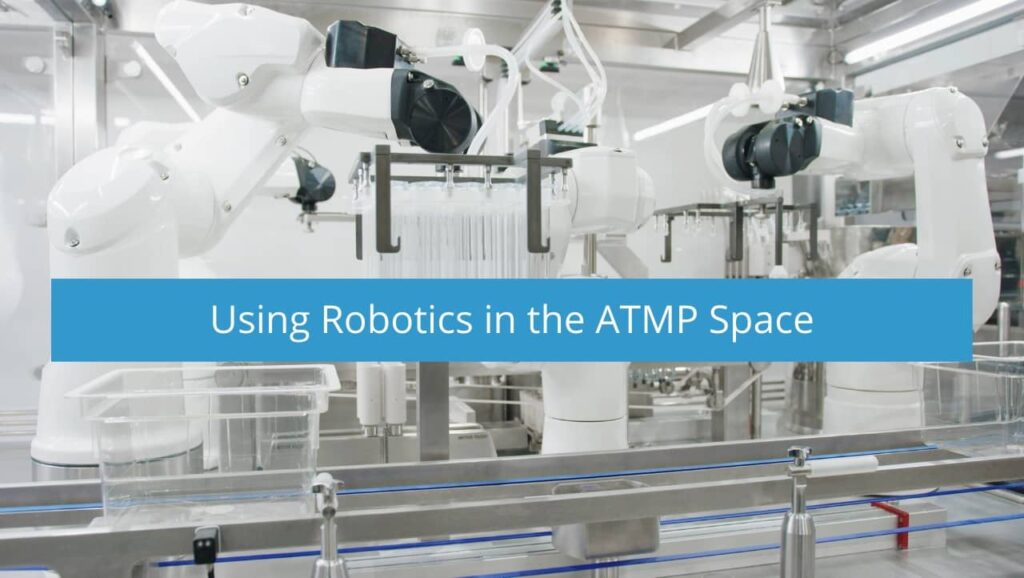
The use of robotics to manufacture Advanced Therapy and Medicinal Products (ATMP) solves many challenges, including significantly limiting exposure to human generated contamination. There are steps to mitigate contamination points but with the strong endorsements recently made within the current revision to Annex I encouraging the use of robotics (Section 8.9) 1, eliminating human interventions should be considered seriously.
