The following article is part two of a blog series that has been adapted from Principal Commissioning, Qualification and Validation Engineer Jason Rossi’s recent article in Cleanroom Technology. Check out part one here.
When setting the scope for the design of a project, then, what are some practical applications of QbD principles? Starting with product requirements and associated risks to quality, teams should be analyzing the necessary risks that need to be mitigated (material transfer, cleanroom personnel, planned and unplanned interventions, airflow movement, etc.) and risks that can be designed out of an operation completely (various human interventions, sterility processing for containers, reusable product-contacting parts, etc.) and assessing those solutions based on their approximation to effort, resources and documentation needed for that QRM-based process.
One observation that’s apparent and an approach I would strongly advocate for based on my time in the field, is a strategy for applying active feedback and continuous updates to all relevant protocols and processes of an operation; if a CCS is expected to be an active, living program, the parameters that inform it (QRM) and solutions that make it a reality (QbD) should be actively and continuously improved and revised. A set cadence with designated resources for assessing risk and the robustness of an operation’s CCS is a must and should be enacted across relevant stakeholder groups, including operations, materials science, tech transfer, etc. Having specialists available to assess microbial and cross-contamination risks and who understand the role of mitigation and QbD strategies is crucial to regulatory compliance and the long-term viability of a project.
One of my earliest experiences in leading a Quality-by-Design facility construction project was for a multiproduct facility in Europe in 2013, where our team completed a comprehensive risk assessment and analysis before building commenced. Every mode of risk, from contamination to the handling of materials to personnel and environmental risks, was designed for. And one of the first issues to resolve when planning a multiproduct facility is not only the mitigation of microbial contamination, but also cross-contamination. Do the products require separate pathways in and out of the facility, or with the right control measures, can they utilize the same airlock? When our team analyzed the question, we considered the scope of having two separate product pathways and the similarity of products (a significant factor), and determined that, with the proper controls and protocols, the risk from utilizing a shared airlock could be successfully mitigated and was a more viable option. This is the type of science-based balance between risk and resources that a comprehensive QbD approach encourages.

The beauty of these principles is that they apply to every aspect of the facility and cleanroom environment. Are your floors suitable for operation? Are they cleanable and able to bear heavy equipment? Are there wall guards in place to protect from damage and subsequent microbial havens that a dent can create? Do your doors open automatically, or do they require bodily contact from cleanroom personnel to open? Will you need utilities and equipment for the sterilization of containers, or will you utilize pre-sterilized RTU containers? What’s the allocation and layout of graded space, and what do solutions like isolators mean for those considerations? A QbD framework provides clarity on every question applicable to a product’s environment and production.
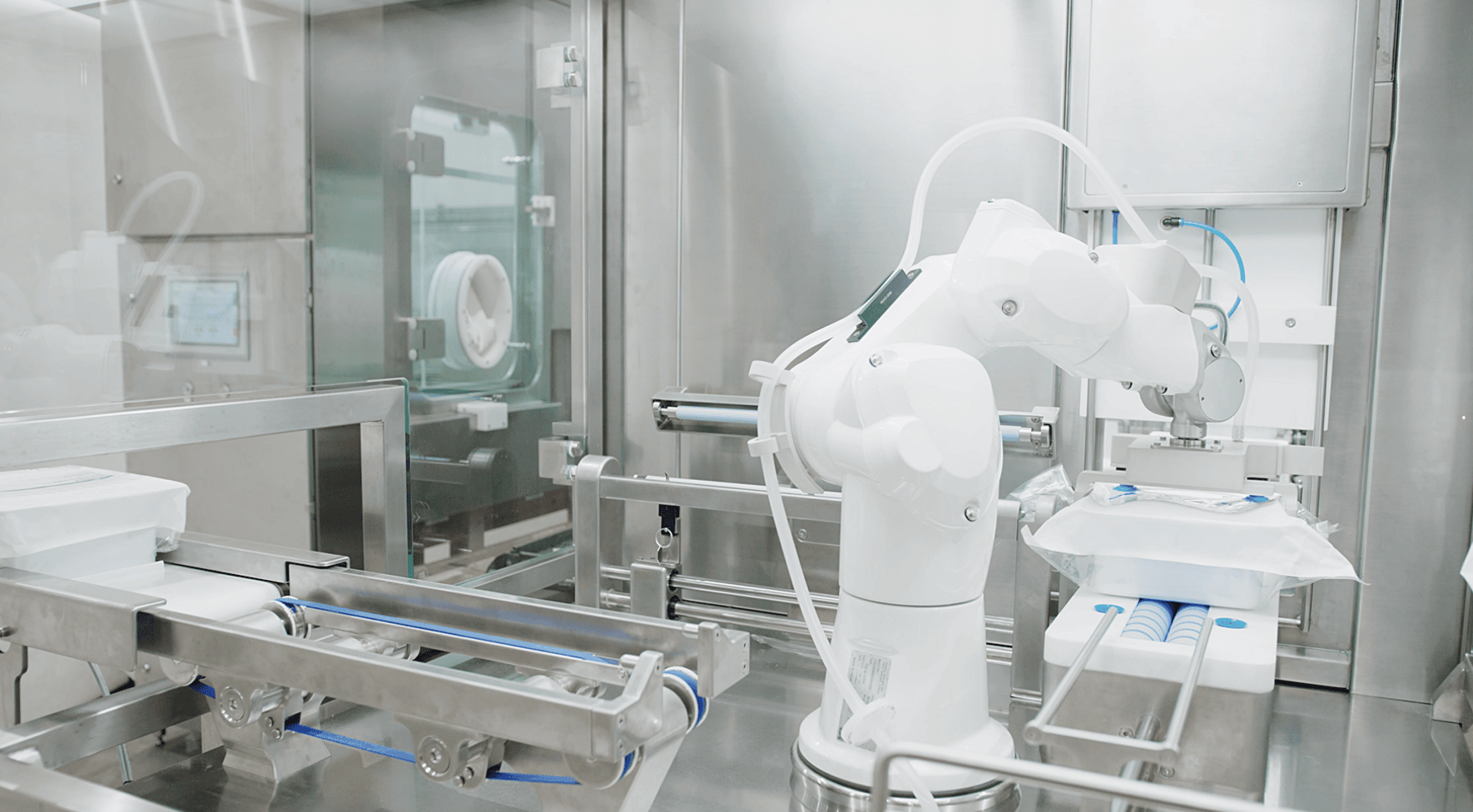
When we arrive at the drug manufacturing process, the benefits of advanced technologies become clear, as does the rationale behind the increased regulatory focus on these solutions. The question in every fill-finish setting is how to best protect the product while eliminating major sources of contamination altogether. This is where, in the past 10-15 years, innovation has taken center stage with the rise of advanced automation, barrier technologies, decontamination methods, and robotics. The use of robotics alone not only eliminates the majority of operator interventions but also eliminates operator variability, which has long been a major risk factor in aseptic operations. Critical zone operations can now be carried out automatically in meticulously controlled Grade A environments, the full potential of which the industry will explore well into the future. As regulators continue to advocate for both a science-based approach to risk and quality, as well as advanced technological solutions, our ability to eliminate risk through sound QRM principles will continue to expand. The key in drug manufacturing settings remains a commitment to science-backed protocols and processes, and an active pursuit of continuous improvement throughout the life of the product.