AST’s VP of Technical Sales, Josh Russell, was interviewed in the June edition of The Medicine Maker as part of a two-part series on trends, best practices, and the future of fill-finish manufacturing.
In part one, essential aspects of sound aseptic processing were discussed as well as considerations for ensuring product integrity, navigating planned and unplanned interventions, and preparing pharmaceutical manufacturers for regulatory and operational success.
Fill-Finish: The Easy Step? Think Again
How much attention do you pay to the fill-finish process? And how much do you know about its importance?
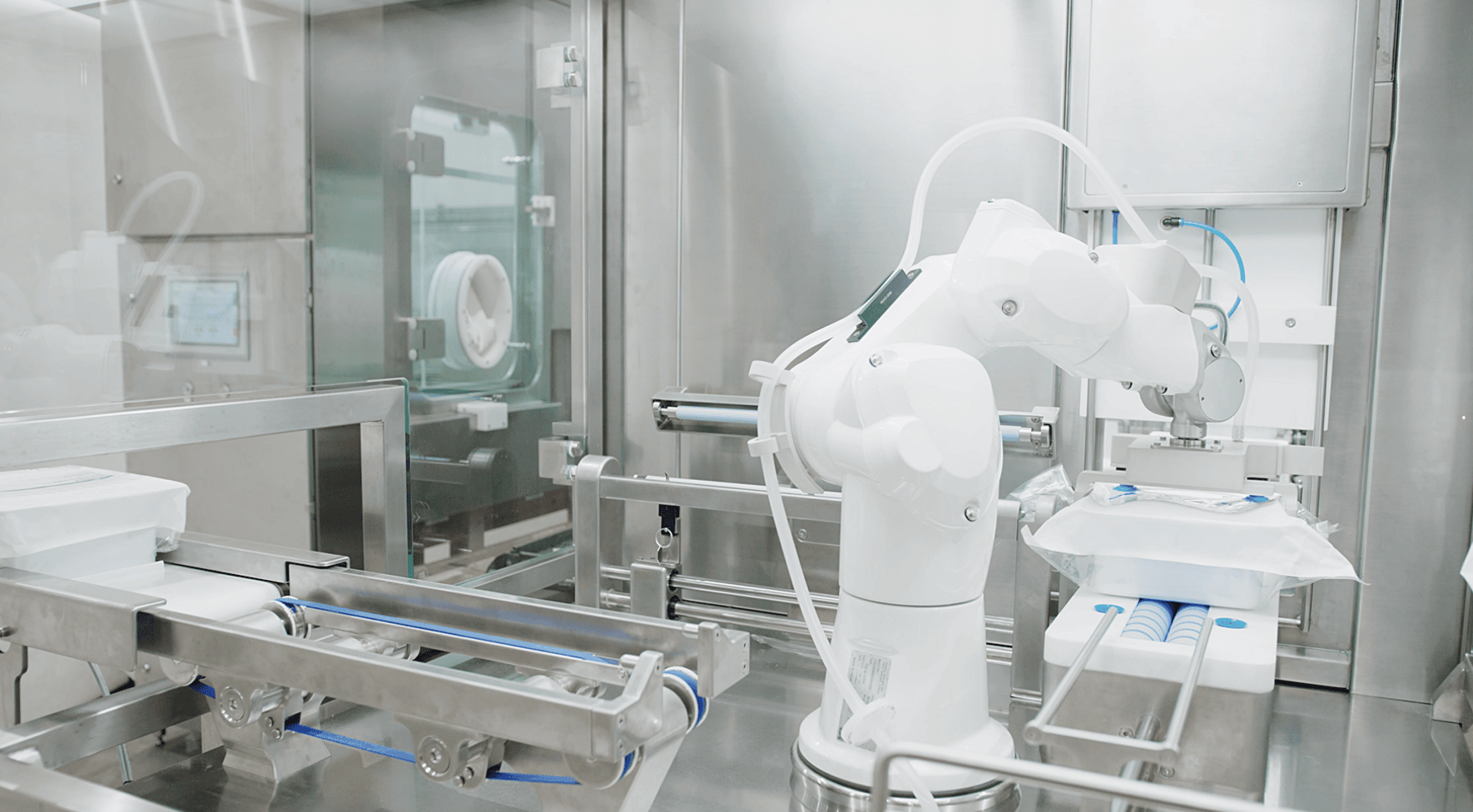
The fill-finish step is the final and one of the most critical stages of the production process, where the drug product – often a sterile liquid – is transferred into its final container, such as a vial, syringe, or cartridge. For lyophilized drugs, the liquid is filled into the container and then freeze-dried in place. This step must be carried out under highly controlled and sterile conditions to ensure the product remains uncontaminated and safe for patient use. Once filled, the containers are sealed, labeled, and packaged, completing the product’s journey from formulation to distribution.
Sounds simple, doesn’t it? However, it’s a very complicated process that can have serious consequences if anything goes wrong. Additionally, because fill-finish requires specialized facilities and equipment, it can become a bottleneck in production, especially during times of high demand or rapid product launches.
In part two of Medicine Maker’s fill-finish roundtable, Josh discusses common operational obstacles in pharmaceutical manufacturing, technological advancements over the past five years, and what the future holds for process and facility design.

Worst-Case Scenarios and Ideal Facilities in Fill-Finish
Is the complexity of the fill-finish process underappreciated in pharma manufacturing? Four experts discuss why it’s important to get fill-finish right and what ideal processes should look like.
Mistakes in fill-finish can lead to project delays, increased costs, and even company failure. In a recent discussion, four experts looked at the perception of fill-finish activities and best practices for success.
Here, we ask four experts to look back on key advances in fill-finish over the last 5 years, worst-case scenarios, and how facilities should look in an ideal world.