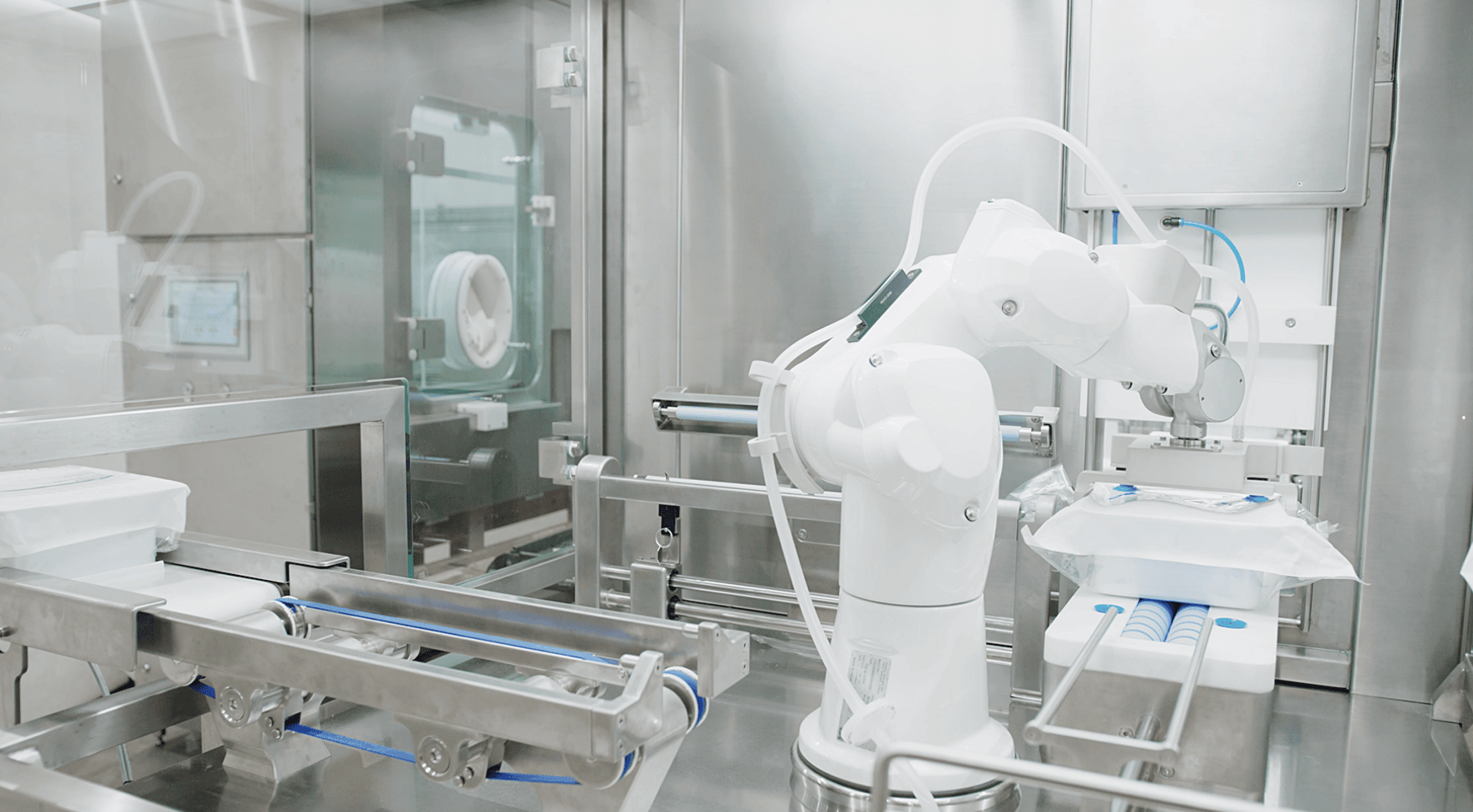
AST Academy

AST Academy offers comprehensive expertise and resources to get your drug product manufacturing operation off the ground and on the road to commercial realization. Equipping drug manufacturers with the training and tools to get the most out of their turnkey solutions from AST, our team of experts is your resource for cGMP guidance and aseptic program development to address the full scope of risk analysis, aseptic design and processing, and CQV needs.
AST Academy training and service offerings are available with all new AST systems. Reach out for a quote today and start your journey toward cGMP production.

Training & Service
AST: Helping pharmaceutical manufacturers navigate the drug product lifecycle.
Training areas:
- Machine Operation
- GMP Documentation
- Audit Preparation
- Aseptic Processing and Basic Microbial Training
Service areas:
- Comprehensive Risk Assessment
- GMP Documentation Packages
- Environmental Program Design
- Commissioning, Qualification, and Validation Program Design
- GMP Facility Design and Manufacturing Strategies

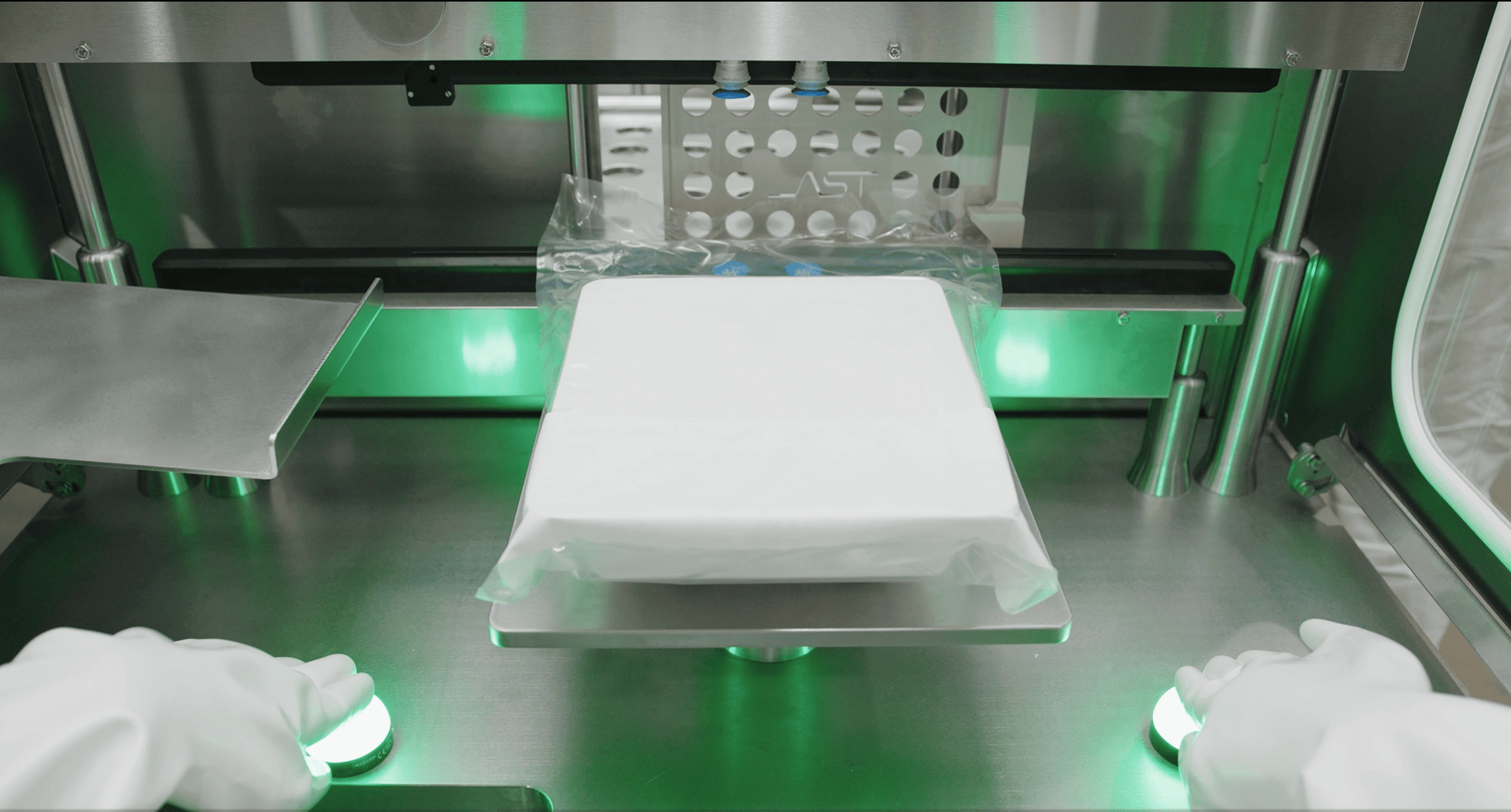
AST Academy – Comprehensive Training, Support, and Service for Drug Product Manufacturing
CQV Roadmap
Get expert support on the road to GMP production. AST provides consultation and trainings on machine qualification planning, preparation, GMP guidance and more to get our equipment validated for GMP use.
Life Science Training and Certification
AST provides in-depth aseptic, skill, and competency training, evaluation, and certifications on machine setup, operations, and basic services.
Aseptic Operations & GMP Services
AST provides consultations and services to optimize your facility and Aseptic process design in line with GMP best practices, including EM program design, operation-wide risk assessment, audit preparation, readiness assessments, and facility design.
SOP JUMPSTART
Fast-track your AST solution with comprehensive machine training, documents, and templates for machine-related SOPs that cover the entire aseptic boundary of the equipment.

Training Programs
The AST Academy is a knowledge hub and training resource designed to be accessible for customers at every skill level. Training areas are available as standalone offerings or combined in the following packages:
Our Aseptic Processing and Basic Microbial Training is a hands-on course that teaches you and your organization basic principles, processes, and systems related to aseptic manufacturing. Course includes:
- Basic microbiology
- Hygiene programs and applications
- Airflow visualization basics and how it plays a role in manufacturing
- Cleanroom terminology and principles
- Grade vs ISO definitions and classifications
- Personnel and gowning procedures
- Fill-finish cGMP procedures and best practices
Services Packages
AST offers a range of CGMP services packages to address documentation, process design, risk assessment, and GMP operational needs. Service areas are available as standalone offerings or combined in the following packages:
- EM program design and implementation
- Smoke study design and execution
- Aseptic process simulation including intervention planning and media fills
- GMP documentation packages including SOPs, Work Instructions, URS and full-range protocol support
AST Launch
AST Launch is a comprehensive, full-service offering designed to get AST fill-finish solutions production-ready and compliant as soon as possible. Customizable to customer needs, AST Launch offers an end-to-end package of training, services, guidance, and documentation designed to bridge the CQV journey and get your facilities, processes, and personnel cGMP-ready.
Benefits:
– Hands-on expert guidance throughout the commissioning, qualification, and validation process
– Faster operational start-up time
– Unified FAT and complete cGMP documentation, including SOPs
– Full Validation and Qualification support of equipment, including PPQ and Pre-approval Inspection support
– Comprehensive QRM and CCS strategy implementation, including multi-product risk assessment and FMEA (Failure Modes Effect Analysis)
– Component Matrix Analysis
– Ongoing technological and regulatory support throughout the life of your equipment
“Whatever the stage of the drug product lifecycle, the integrity of the product and surrounding processes is always the priority in an aseptic processing operation. Our team at AST is with you every step of the way to ensure our equipment is installed successfully and production-ready as soon as possible.”
- jason rossi

Jason Rossi, Principal Commissioning, Qualification and Validation Engineer