PRODUCTS
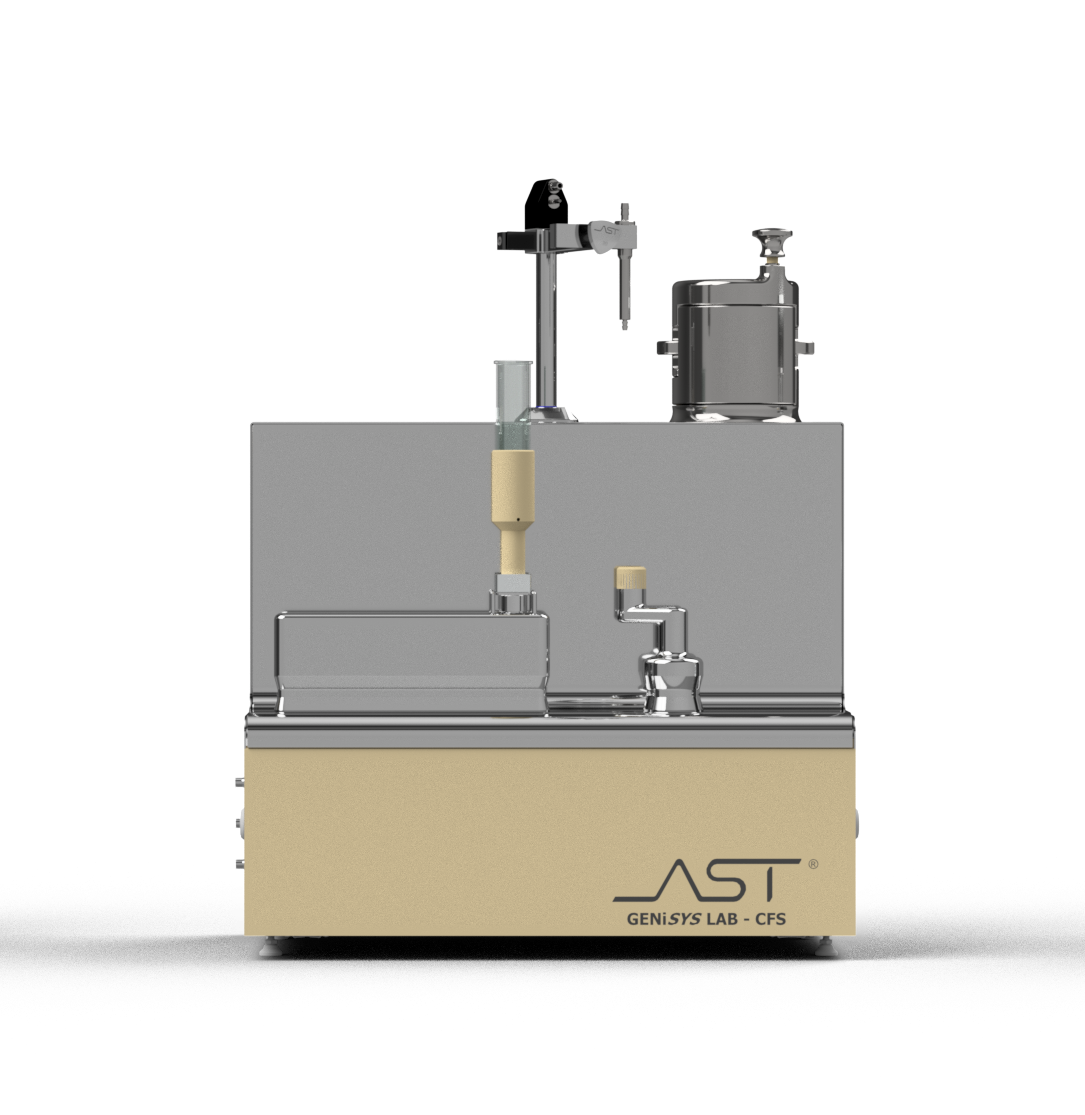
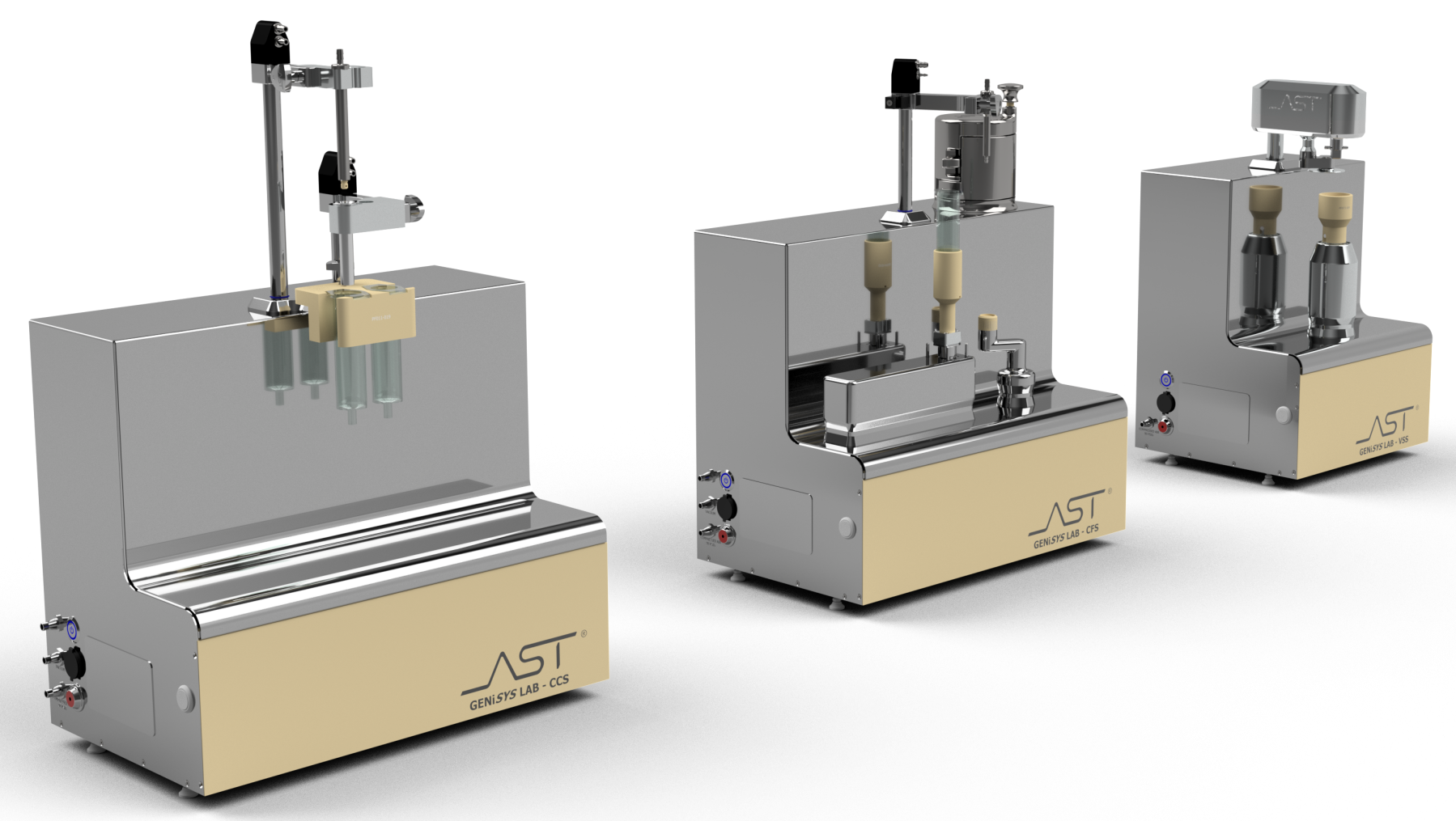
GENiSYS® Lab: Tabletop Innovation for Aseptic Processing
AST’s GENiSYS Lab series offers automated, flexible aseptic processing (container filling, container closing, and vial sealing). These advanced systems were designed for laboratory settings and configured with a tabletop technology that leverages precise automation for critical points in aseptic operation. The GENiSYS Lab solutions lower overall risk and ensure the highest standards of product quality and data integrity from the very start of drug development. User-friendly operations, executed through a portable HMI, utilize flexible recipe-driven production to meet the full scope of small-scale drug processing, including research and development, process development, and commercial production of highly targeted liquid pharmaceuticals.
In the Know
Capabilities
- Proven and standardized tabletop solutions ideal for small-scale drug development
- Universal RTU container processing capabilities
- cGMP-proven design and technology
- Scalable solutions featuring turnkey technology available on all our advanced systems
- Easy validation and efficient technology transfer
- Available with in-process controls for quality control and high-yield operations
- Full ramp-up support including qualification services and full documentation packages
- Responsive, 24/7 aftermarket support throughout the life of your equipment
- Toolless change parts
- Processes vials, syringes, and cartridges.
- Configurable, recipe-driven production and process monitoring.
- Centralized, remote HMI for operation and data retention, and optional EBR system for 21 CFR Part 11 Compliance
Drug developers frequently revisit the question of what ways are available to make laboratory drug processing more efficient and effective. Automation is one avenue that continues to grow in application and potential for drug discovery and development. Though benchtop operations are vital to the drug development journey, many laboratory settings still use primarily manual methods. Automated solutions address and correct the inefficiencies and obstacles associated with a manual-only approach, including human errors, contamination risks, and incomplete data.

Module by Module
Highlights:
- Closes cartridges and syringes
- Inert gas purging
- Precise piston placement
- Vacuum, vent-tube or combination stoppering
- Available in two-up configuration
- Multi-stage vacuum insertion available
Highlights:
- Accurate dispense system
- Process flexibility allows the system to fill
- Inert gas purging during filling
- Optional in-process weight check
- Optional product bag with surge stand
- Available with peristaltic or Rotary piston pumps
- For vials: stoppering with serum or lyo stopper or press-fit cap
Highlights:
- Provides aesthetic pharmaceutical-grade seal
- Caps vials and bottles
- Gentle, free-spinning disk sealing for lower particle generation
- Eliminates crimp-style inconsistencies
You've seen the GENiSYS® Lab in motion...
Perhaps it's time to discover how it would work with your application?
Meeting Industry Needs | Applications

Research and development is a critical undertaking for pharmaceutical manufacturers and is the backbone of the life sciences industry. Every lifesaving and life-improving treatment that has ever been produced started in a laboratory setting. Before a treatment can reach a patient in need, it’s in the laboratory that it’s discovered, refined, and then pushed forward to start its pre-clinical to commercial journey.



Use Cases

A global biomanufacturing company partnered with AST on an innovative, isolated GENiSYS® Lab systems for processing cell therapy products.
Download the Case Study